TMS: A Useful Clinical Tool for Treatment-Resistant Depression
How can transcranial magnetic stimulation help patients with treatment-resistant depression?
Tohey Vector/Adobe Stock
Developed in 1985 and approved by the US Food and Drug Administration in 2008, transcranial magnetic stimulation (TMS) technology has been gaining clinical interest as an effective treatment option for patients with treatment-resistant depression. Two-thirds of TMS patients experienced either full remission of their depression symptoms or noticeable improvements.1 TMS is a noninvasive outpatient procedure without serious adverse effects, making it an attractive option for patients who have not found relief from other treatments, including psychopharmacology and psychotherapy.
How TMS Works
TMS uses an alternating current passed through a metal coil placed against the scalp to generate rapidly alternating magnetic fields. These pass through the skull nearly unimpeded and induce electric currents that depolarize neurons in a focal area of the surface cortex.
The magnetic field generated by TMS is comparable to that of a standard magnetic resonance imaging device (MRI), measured at approximately 1.5 to 3 Teslas. However, the TMS field is focal (beneath the coil), whereas the MRI field is large and fills the room housing the MRI device.
One hypothesis on how TMS works is that the stimulation of discrete cortical regions alters pathologic activity within a network of gray matter brain regions, specifically those involved in mood regulation and connected to the targeted cortical sites.2 Functional imaging studies support this hypothesis by showing TMS can change activity in brain regions remote from the site of stimulation.3,4
TMS has many molecular effects comparable to electroconvulsive therapy (ECT), including increased monoamine turnover and normalization of the hypothalamic pituitary axis.5 However, unlike ECT, TMS does not require general anesthesia and is not associated with impaired cognition. Additionally, in one neuroimaging study of depressed patients, a prefrontal serotonin deficiency at baseline normalized after treatment with TMS.
High-frequency stimulation is thought to excite the targeted neurons and is typically used to activate the left prefrontal cortex. Low-frequency stimulation appears to inhibit cortical activity and is usually directed at the right prefrontal cortex.
Consistent with this hypothesis, a review examined 66 studies in patients with depression who were treated with TMS targeting the dorsolateral prefrontal cortex. It found that high-frequency TMS generally increased regional cerebral blood flow, whereas low-frequency TMS generally decreased regional cerebral blood flow, which is reduced in a depressed brain.6
When Is TMS a Good Option?
TMS is indicated for patients with unipolar major depression who have failed at least 1 antidepressant medication. In addition, TMS is indicated for patients who responded to a prior course of TMS.7 Its use for these patients is consistent with treatment guidelines from the American Psychiatric Association, Canadian Network for Mood and Anxiety Treatments, and the Royal Australian and New Zealand College of Psychiatrists.
The patient evaluation should confirm the primary diagnosis of treatment-resistant depression and determine whether the TMS intervention can be used safely. The assessment includes examinations of psychiatric history, general medical history, physical health, and mental status with emphasis upon depressive symptoms. This should emphasize risk factors for seizures and preexisting neurologic disease, such as epilepsy, intracranial masses, and vascular abnormalities.
TMS is contraindicated in patients with: increased risks for seizures, implanted metallic hardware (aneurysm clips, bullet fragments, etc), cochlear implants, implanted electrical devices (pacemakers, intracardiac lines, medication pumps, etc), and unstable general medical disorders. The Figure shares a 12-item questionnaire to determine if a patient is a good TMS candidate.
Figure. Screening Questions for TMS Candidates24

How Effective Is TMS?
Multiple reviews have found consistent evidence that TMS provides a clinically relevant benefit to patients with treatment-resistant depression. In patients with acute major depression who have not responded to at least 1 antidepressant medication, numerous meta-analyses of randomized trials have found TMS superior to placebo treatment.8-11 It is not known if maintenance treatment with TMS for unipolar major depression is beneficial.
A meta-analysis of 34 randomized trials compared TMS with placebo treatment in 1383 patients with treatment-resistant major depression. It found that improvement was greater with active treatment.12 Add-on treatment with TMS was efficacious in patients who had not responded to an adequate antidepressant therapy. Response (for example, the reduction of baseline symptoms ≥ 50%) occurred in more patients who received active (47%) versus placebo (22%) TMS.12
There have been no consistent predictors of response according to a meta-analysis. A 1-year, prospective observational study of 120 patients who responded or remitted with acute TMS found that the durability of response to TMS was not associated with age, sex, severity of depressive symptoms prior to TMS, nor the number of failed antidepressant trials prior to TMS.13
For treatment of major depression, TMS is less efficacious than ECT. Follow-up studies of patients with major depression who were treated acutely with TMS in randomized trials indicate that the short-term benefits of TMS are stable.14 With regard to longer-term benefits of TMS, prospective, observational studies lasting at least 6 months suggest that in patients with major depression who improve with acute TMS, relapse occurs in about 35%.15
For patients with unipolar major depression who improve with a course of TMS and subsequently deteriorate or relapse, reintroduction of TMS using the same stimulation parameters may be helpful.16 It is not known if maintenance treatment with TMS for unipolar major depression is beneficial, as few randomized trials using standard protocols have been conducted. However, in several small, observational studies of patients, the results suggest that maintenance TMS may, perhaps, be beneficial.7
How Safe Is TMS?
TMS is generally safe and well-tolerated. For example, in a randomized trial of 301 patients, investigators found that study discontinuation due to adverse effects was comparable for active and placebo TMS (5% and 3%, respectively).17
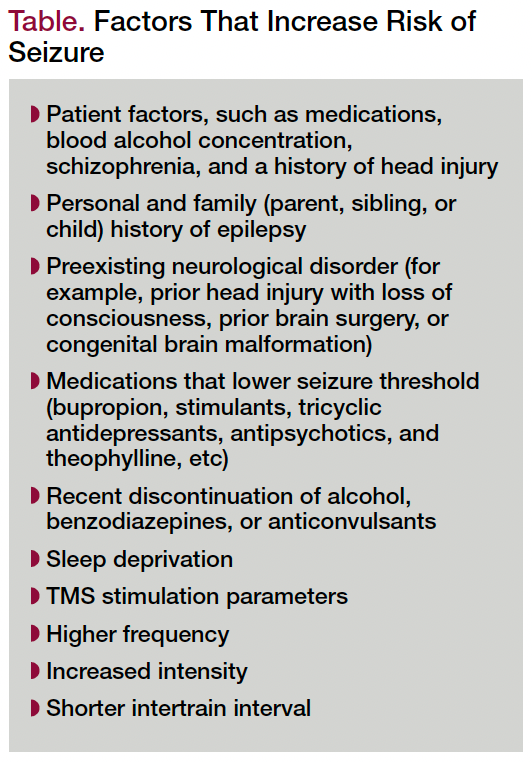
The most serious adverse effect of TMS is a generalized tonic-clonic seizure. However, the risk of seizure appears to be comparable to that for anti-depressant medications. Seizures probably occur in less than 0.1 to 0.5% of patients when safety guidelines are followed regarding patient selection and stimulation parameters. Seizures that have occurred were self-limited, required no medications, and did not recur.18 Factors that increase the risk of seizures can be found in the Table.
Table. Factors That Increase Risk of Seizure
Other effects include hypomania and mania, described in randomized trials17 and reports of patients with major depression (both unipolar and bipolar) who were treated with TMS.19,20
However, the clinical significance is not known, because patients with bipolar major depression can switch to mood-elevated states in the absence of an antidepressant treatment. Treatment of unipolar major depression with TMS does not appear to increase suicidal ideation or behavior.21
What About Side Effects?
Headache and scalp pain. A review of randomized trials in patients with major depression found that the incidence of headache with active treatment and placebo treatment was 28% and 16%, respectively. The incidence of scalp pain with active and placebo treatment was 39% and 15%, respectively. No migraine headaches have been reported. Headache and scalp pain may be more pronounced when higher stimulation frequencies and intensities are used. Topical lidocaine may reduce scalp pain. Reducing stimulation intensity can decrease scalp discomfort, but this can also reduce efficacy of treatment. For sensitive patients, the dose of TMS can be titrated up during the first week. Headache and scalp pain generally resolve over the first 2 weeks, although some patients may require an analgesic, such as acetaminophen or ibuprofen.22
Transient (< 4 hours) increase in auditory threshold. This is caused by repeated clicks that are produced as current passes through the stimulating magnetic coil and mechanically deforms the coil. Hearing loss is prevented with foam earplugs or noise protection ear coverings.
Vasovagal syncope. Management generally consists of reassurance.
Special Concerns
Elderly. For older patients with major depression, TMS can be beneficial if the stimulation intensity is sufficient.17 Prefrontal atrophy in older patients can increase the distance between the coil and cortex to the point that lower-intensity stimulation, which typically penetrates to a depth of 2 to 3 cm, does not affect cortical activity. Increasing the intensity above the motor threshold can overcome the added distance.5
Poststroke depression. Depression frequently occurs after stroke, and TMS may help these patients.
Pregnancy and postpartum depression. For these patients with major depression, observational studies suggest that TMS may possibly be safe and effective. It appears unlikely that the fetus is directly affected by TMS because magnetic fields rapidly attenuate with distance.23
Concluding Thoughts
TMS is an exciting and promising therapy that can provide real and lasting relief for patients with treatment-resistant depression. Conducted in an outpatient setting, TMS is a noninvasive procedure that is generally safe and well-tolerated. This provides patients with the flexibility to seek treatment in a way that does not disrupt their daily lives. TMS has an equally promising future, with studies exploring its expanded applications, as well as its use as an ongoing maintenance treatment.
Dr Ramanujam is a psychiatrist and regional medical director at Mindpath Health.
References
1. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587-596.
2. Baeken C, De Raedt R. Neurobiological mechanisms of repetitive transcranial magnetic stimulation on the underlying neurocircuitry in unipolar depression. Dialogues Clin Neurosci. 2011;13(1):139-145.
3. Kito S, Fujita K, Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58(1):29-36.
4. Fitzgerald PB, Sritharan A, Daskalakis ZJ, et al. A functional magnetic resonance imaging study of the effects of low frequency right prefrontal transcranial magnetic stimulation in depression. J Clin Psychopharmacol. 2007;27(5):488.
5. George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. 2011;168(4):356-364.
6. Noda Y, Silverstein WK, Barr MS, et al. Neurobiological mechanisms of repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex in depression: a systematic review. Psychol Med. 2015;45(16):3411-3432.
7. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346.
8. Schutter DJ. Quantitative review of the efficacy of slow-frequency magnetic brain stimulation in major depressive disorder. Psychol Med. 2010;40(11):1789.
9. Berlim MT, Van den Eynde F, Jeff Daskalakis Z. Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology. 2013;38(4):543.
10. Berlim MT, Van den Eynde F, Daskalakis ZJ. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol Med. 2013;43(11):2245.
11. Allan CL, Herrmann LL, Ebmeier KP. Transcranial magnetic stimulation in the management of mood disorders. Neuropsychobiology. 2011;64(3):163-9.
12. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
13. Schutter DJ. Antidepressant efficacy of high-frequency transcranial magnetic stimulation over the left dorsolateral prefrontal cortex in double-blind sham-controlled designs: a meta-analysis. Psychol Med. 2009;39(1):65.
14. Kedzior KK, Reitz SK, Azorina V, Loo C. Durability of the antidepressant effect of the high-frequency repetitive transcranial magnetic stimulation (rTMS) in the absence of maintenance treatment in major depression: a systematic review and meta-analysis of 16 double-blind, randomized, sham-controlled trials. Depress Anxiety. 2015;32(3):193.
15. Avery DH, Holtzheimer PE, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59(2):187-194.
16. Liu B, Zhang Y, Zhang L, Li L. Repetitive transcranial magnetic stimulation as an augmentative strategy for treatment-resistant depression, a meta-analysis of randomized, double-blind and sham-controlled study. BMC Psychiatry. 2014;14:342.
17. Xia G, Gajwani P, Muzina DJ, et al. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2008;11(1):119-130.
18. Stultz DJ, Osburn S, Burns T, et al. Transcranial Magnetic Stimulation (TMS) safety with respect to seizures: a literature review. Neuropsychiatr Dis Treat. 2020;16:2989-3000.
19. Dolberg OT, Schreiber S, Grunhaus L. Transcranial magnetic stimulation-induced switch into mania: a report of two cases. Biol Psychiatry. 2001;49(5):468-70.
20. Garcia-Toro M. Acute manic symptomatology during repetitive transcranial magnetic stimulation in a patient with bipolar depression. Br J Psychiatry. 1999;175:491.
21. Abdelnaim MA, Langguth B, Deppe M, et al. Anti-suicidal efficacy of repetitive transcranial magnetic stimulation in depressive patients: a retrospective analysis of a large sample. Front Psychiatry. 2020; 10:929.
22. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
23. Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008-2039.
24. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire TMS: an update. Clin Neurophysiol. 2011;122:1686. ❒

