Bespoke Psychopharmacology for Bipolar Disorder: An Individualized Patient Approach
The author discusses evidence-based treatment, highlighting clinical trials that support specific uses and patterns of efficacy.
FreshIdea/AdobeStock

CONFERENCE REPORTER
“It is much more important to know what sort of a patient has a disease
than what sort of disease a patient has”
- Sir William Osler
Imagine devising a pharmacotherapy regimen for each and every patient you see with the same kind of individualized precision and artisanal craftsmanship that an expert tailor uses to create a bespoke suit of clothing. Clinicians sometimes merely equate “personalized medicine” with biomarkers (such as pharmacogenetics), dismissing most other aspects of pharmacotherapy decision-making as largely a trial-and-error process. Yet, when one considers the numerous other clinical, demographic and psychosocial factors that impinge on how any given psychiatric disorder presents, it is not hard to reframe Sir William Osler’s aphorism about “what sort of patient has a disease” as a reckoning of clinical diversity (or what geneticists might call phenotypic heterogeneity).
Rather than simply matching a treatment (say, lithium or divalproex) to a disease state per se (say, bipolar mania), modern psychopharmacology can draw in a much more nuanced fashion from randomized trial data to predict the chances for success with one treatment (or combination) relative to another, based on the presence or absence of key clinical characteristics. “Tailored” pharmacotherapy, informed by the clinical trials literature, involves assessing patient-specific characteristics in order to make reasonable predictions about what treatment or drug combinations have clear rationales and likelihoods for successful outcomes for any one given patient, rather than for a broad diagnosis per se.
In the case of bipolar disorder, clinical diversity is more common than rare. Consider its multitude of contributing factors: patients can manifest pure or mixed mood states, with persistent or undulating symptoms, varying levels of severity and residual symptoms, with or without psychosis, often colored by comorbid medical or psychiatric conditions and personality traits. Bipolar I versus II disorders may differ in symptom profiles (as in depression-proneness or suicide risk) and treatment response (as in the case of antidepressants1).
Some affective or psychomotor symptoms may be more prominent than others; and features such as impulsivity, aggression, suicidality, trauma, cognitive dysfunction, and circadian rhythm disturbances often pose their own conspicuous treatment targets. Tailored approaches also take into account the impact of factors called mediators that can either enhance or derail an otherwise efficacious treatment once it is begun—such as drug adverse effects, treatment adherence, drug-drug interactions, new substance misuse, strength of the therapeutic alliance, or loss of patient insight about the need for ongoing treatment. Biostatisticians call baseline factors that can influence outcome moderators. Examples are described in Table 1.
Profiling patient candidacy for a given treatment
From randomized trials, one can take away a number of general principles to help guide the tailoring process of constructing a “profile” for optimal candidacy with 1 treatment relative to another. For example:
• Divalproex may yield a better response than lithium in mania involving mixed (rather than pure manic) episodes,4 multi-episode presentations,5 and comorbid alcohol use disorders.6
• The combination of lithium plus divalproex seems better than divalproex alone in rapid cyclers7 or during relapse prevention following a manic episode.8
• Comorbid anxiety symptoms have been shown to improve during treatment for bipolar depression with each of the four existing FDA-approved agents for bipolar depression. Co-therapy with gabapentinoids offers a rationale-based further anxiolytic approach. With the exception of paroxetine, the anxiolytic properties of SSRIs in bipolar patients are largely unknown and cannot be assumed.
• Lithium may warrant independent consideration for its apparent anti-impulsivity effects in patients at risk for acting on suicidal thoughts.
• Among second-generation antipsychotics (SGAs), there is tremendous variability in efficacy (or lack thereof) for bipolar depression. Aripiprazole, ziprasidone, and risperidone have only negative randomized trials in bipolar depression. Relative to quetiapine or olanzapine-fluoxetine combination, lurasidone and cariprazine are the more weight- and metabolically neutral SGAs FDA-approved for bipolar depression; all 4 exert large effects in bipolar depression, so how else do they differ? Lurasidone lacks data in mania and failed to show value for relapse prevention, but it has demonstrated cognitive benefits.9 Cariprazine, by contrast, treats both manias and depressions and its high affinity for D3 dopamine receptors makes it an attractive option in the setting of low motivation and reward. Its potential value for relapse prevention is unknown. Both lurasidone and cariprazine pose concerns for dose-related akathisia and EPS, suggesting that low doses often may be more useful and tolerable than high doses.
• Beyond 6 months after a manic episode, continuing an SGA plus mood stabilizer may add no greater value for relapse prevention than an antimanic mood stabilizer alone—but incur higher metabolic liability.10
How trial designs tweak the pharmacology evidence-base
Understanding how clinical trial designs affect study findings is another key aspect of using evidence-based data when crafting a bespoke treatment regimen. An important example involves recognizing low baseline severity as a moderator that can inflate placebo response and obscure true drug advantages. One of the best illustrations of this was the failure of lamotrigine to outperform placebo in FDA trials of acute bipolar depression—likely not so much because the drug was ineffective (and having nothing at all to do with lamotrigine’s titration schedule), but rather, because the placebo response rate was inordinately high among low baseline severity patients.11 By contrast, in patients with high baseline severity, a much lower placebo response rate allowed clear separation of drug effect from placebo.
So what does this mean for the practitioner, who is otherwise left thinking only that lamotrigine is just “a mood stabilizer” that’s sometimes used off-label to treat bipolar depression? Quite a bit: the evidence base for lamotrigine shows that it does exert efficacy in bipolar depression, but the unexpectedly high placebo response rate in pivotal trials (due at least partly to low baseline severity) clobbered any meaningful difference between it and active drug. Meanwhile, other studies have shown lamotrigine to synergize with lithium or quetiapine, consequently relegating it to an often more ambiguous role in treating acute bipolar depression.
Antidepressants: bespoke or “unspoke”?
What about antidepressants in bipolar depression—an area that remains mired more in controversy and strong opinion than extensive evidence? Most modern antidepressants (those developed after 1999) have never been formally studied versus placebo in bipolar depression, making them truly experimental options. Much-publicized concerns that antidepressants are mainly “hazardous” for inducing mania or cycle acceleration run counter to prospective studies that report only about a 12% incident rate of antidepressant-induced mania.1
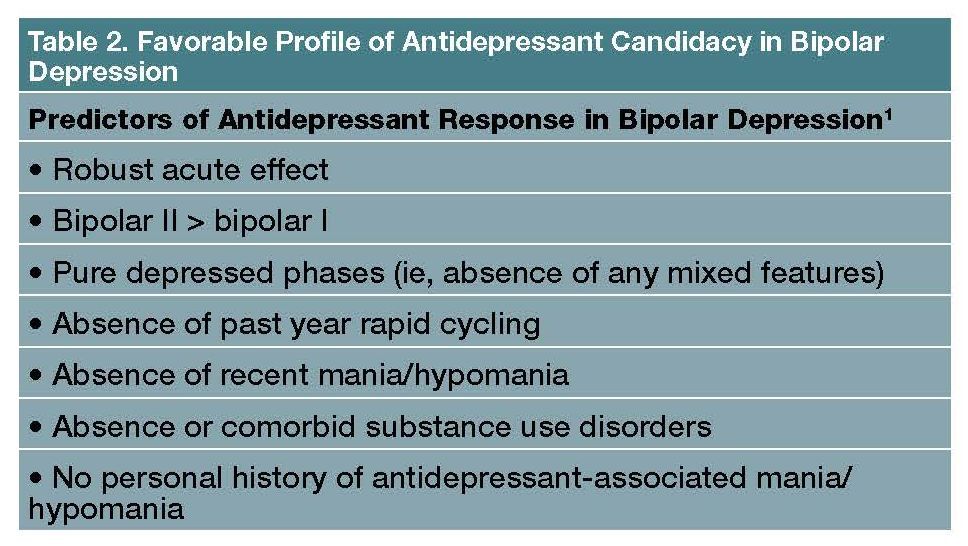
Popular admonitions that antidepressants as a class are ineffective for bipolar depression come from scant placebo-controlled trial literature involving mostly older studies of paroxetine. Fluoxetine (either combined with lithium or olanzapine, or even as monotherapy in bipolar II depression) and sertraline (in bipolar II depression; reviewed by Goldberg1) actually both are among the more evidence-based treatment options in bipolar depression. Antidepressant “candidacy” in bipolar depression is favored by the profile of characteristics listed in Table 2.
Perhaps the strongest moderator of a successful outcome is a marked acute response, which tends to predict a better enduring effect with minimal risk for mood destabilization; by contrast, a partial or weak acute response bodes poorly. Importantly, less than one-third of patients with bipolar depression display strong antidepressant responses to modern studied antidepressants (ie, sertraline, venlafaxine, or bupropion). And, similar to unipolar depression, chances for responsivity drop precipitously with each successive failure.
Final considerations: pharmacological parsimony
A third or more of individuals with bipolar disorder take 3 or more psychiatric drugs;12 therefore, let each one count purposefully. Elegance comes from targeting multiple treatment domains with the fewest agents or capitalizing on known synergies while avoiding mechanistic redundancies. Divalproex, for instance, may represent the treatment of choice in a mania-prone male patient with migraine headaches, impulsive aggression, and alcohol use disorder.
Bupropion could be a handy intervention for a patient with bipolar II pure-depression, prone to nonmixed depression-polarity who is also overweight, smokes, and has attentional complaints. (Ar)modafinil or lisdexamfetamine added to an antimanic drug may neatly address bipolar depression, anergia, and attentional complaints, while the latter may additionally help to manage binge eating or overweight. Every component of a treatment regimen should have a definable purpose; agents deemed ineffective after an adequate trial warrant deprescribing; and decisions about sequential pharmacotherapies are in turn best guided by a careful assessment of patients’ moderating features alongside an informed knowledge of the existing clinical trials literature.
Dr Goldbergis clinical professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York. The author reports the following disclosures: Abbvie Pharmaceuticals: Speakers Bureau; American Psychiatric Publishing: Royalties; BioXCel: Consultant; Intra-cellular Therapies: Speakers Bureau; Neurocrine: Consultant; Otsuka: consultant; Sage Pharmaceuticals: Consultant; Sunovion: Consultant, Speakers Bureau. Dr Goldberg spoke at PsychCongress 2020 in a presentation titled “Tailoring Individualized Pharmacotherapy for Bipolar Disorder: How to Translate Findings from Clinical Trials to a Single Patient.”
References
1. Goldberg JF. Determining patient candidacy for antidepressant use in bipolar disorder. Psych Ann. 2019;49:386-391.
2. Sajatovic M, Forester BP, Tsai J, et al. Efficacy oflurasidonein adults aged 55 years and older with bipolar depression: post hoc analysis of 2 double-blind, placebo-controlled studies. J Clin Psychiatry. 2016;77(1):e1324-e1331.
3. Grof P, Duffy A, Cavazzoni P, et al. Is response to prophylactic lithium a familial trait? J Clin Psychiatry. 2002;63(10):942-947.
4. Swann AC, Bowden CL, Morris D, et al. Depression during mania: treatment response to lithium or divalproex. Arch Gen Psychiatry. 1997;54:37-42.
5. Swann AC, Bowden CL, Calabrese JR, et al. Differential effect of number of previous episodes of affective disorder on response to lithium or divalproex in acute mania. Am J Psychiatry. 1999;156(8):1264-1266.
6. Salloum IM, Cornelius JR, Daley DC, et al. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2005;62(1):37-45.
7. Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry. 2005;162: 2152-2161.
8. Geddes JR, Goodwin GM, Rendell J, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised, open-label trial. Lancet. 2010; 375(9712):385-395.
9. Yatham LN, Mackala S, Basivireddy J, et al. Lurasidone versus treatment as usual for cognitive impairment in euthymic patients with bipolar I disorder: a randomised, open-label, pilot study. Lancet Psychiatry. 2017;4:208-217.
10. Yatham LN, Beaulieu SchafferA, et al. Optimal duration of risperidone or olanzapine adjunctive therapy to mood stabilizer following remission of a manic episode: a CANMAT randomized double-blind trial. Mol Psychiatry. 2016;21:1050-1056.
11. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry. 2009;194:4-9.
12. Goldberg JF. Complex combination pharmacotherapy for bipolar disorder: knowing when less is more or more is better. FOCUS. 2019;17:218-231.