Augmentation Strategies for Treatment-Resistant Depression
Given that two-thirds of patients treated for a major depressive episode will fail to achieve remission of symptoms after 2 or more treatment trials of first-line antidepressants, the probability of remission will further decrease with subsequent medication trials. Treatment strategies for patients with TRD include augmentation, where a medication is added to a current antidepressant versus switching to a different antidepressant.
LFK/AdobeStock

SPECIAL REPORT: TREATMENT-RESISTANT DEPRESSION
Many patients presenting with unipolar major depression do not achieve remission after their initial treatment. The term treatment-resistant depression (TRD) typically refers to major depression that does not remit after 2 antidepressant trials of adequate dose and duration; however, the definition has not been standardized.1,2
Several staging models have been proposed over the years for classifying and defining TRD. The Thase and Rush Model is the most widely used in TRD. In this model, failure to respond to 1 adequate antidepressant trial from a major antidepressant class is considered stage I TRD, and those who then do not respond to a second adequate antidepressant trial (from a different class than the antidepressant used in stage I) are termed stage II TRD.3 Several guidelines or staging methods outline specific requirements (eg, the number of adequate trials, dosage, duration, and types of agents) that must be met prior to the patient’s diagnosis as treatment resistant. These methods vary in the degrees of resistance described.4 Other staging methods include the European Staging Model, the Massachusetts General Hospital Staging Model, and the Maudsley Staging Model.5 The overarching staging criteria propose a higher degree of treatment resistance with more treatment failures.
Across different studies of patients with unipolar major depression who receive initial treatment, the prevalence of treatment resistance ranges from approximately 30% to 70%.6-8 Evidence that supports a categorical definition based upon 2 treatment failures includes results from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study (NCT00021528), which prospectively administered up to 4 sequential trials of pharmacotherapy to 3671 patients who presented with unipolar major depression. The rate of remission appeared to be comparable for the initial and second course of treatment (37% and 31%, respectively), and then to decline more substantially for the third and fourth steps of treatment (14% and 13%, respectively).9
Augmentation Strategies
Given that two-thirds of patients treated for a major depressive episode will fail to achieve remission of symptoms after 2 or more treatment trials of first-line antidepressants,9 the probability of remission will further decrease with subsequent medication trials.10 Treatment strategies for patients with TRD include augmentation, where a medication is added to a current antidepressant versus switching to a different antidepressant.
Augmentation strategies can improve treatment response in those who have had a partial response to an initial antidepressant trial. Several clinical trials have examined the relative effectiveness of augmentation of existing antidepressants versus switching to antidepressant monotherapy.11-13 The STAR*D trial revealed that bupropion augmentation and switching to monotherapy were as effective as other treatment strategies.11 The Veterans Affairs Augmentation and Switching Treatment for Improving Depression Outcomes (VAST-D) trial (NCT01421342) showed that augmentation with either aripiprazole or bupropion was more effective than switching to monotherapy with bupropion.12 Similarly, the Optimizing Outcomes of Treatment-Resistant Depression in Older Adults (OPTIMUM) study (NCT02960763) showed that augmentation with aripiprazole was significantly more effective than switching to bupropion monotherapy in achieving remission of depressive symptoms in older adults with TRD.13
The selection of an augmenting agent may depend on patients’ depressive symptoms and clinical presentation. Bupropion is a norepinephrine and dopamine reuptake inhibitor commonly used as monotherapy and adjunct therapy in TRD, but may lower seizure threshold.14 In patients endorsing insomnia, poor appetite, weight loss, and anxiety, augmentation with mirtazapine, a noradrenergic and a serotonergic antidepressant, may be preferred.15 Meanwhile, lithium augmentation, due to its serotonergic properties, is efficacious in depressed nonresponders but requires monitoring of blood levels. The target blood level of lithium for antidepressant treatment ranges from 0.3 to 0.6 mEq, with lithium levels checked every 6 to 12 months.16
Augmentation with second-generation antipsychotics has also exhibited efficacy in the treatment of TRD, thought to be mediated through dopamine receptors.17 Aripiprazole, brexpiprazole, and quetiapine have been approved by the US Food and Drug Administration (FDA) for adjunctive therapy in treating depression. Aripiprazole augmentation has been studied in older adults and was more effective than placebo.18 To illustrate further, a pooled analysis of 16 randomized trials compared adjunctive aripiprazole, olanzapine, quetiapine, or risperidone with placebo in 3480 patients with nonpsychotic, unipolar major depression who failed at least 1 course of antidepressant monotherapy and showed that remission occurred in more patients who received an adjunctive antipsychotic compared with placebo (31% versus 17%, respectively).19
Role of Psychotherapy
Another alternative is to switch from pharmacotherapy to psychotherapy (eg, cognitive-behavioral therapy) or retain the initial antidepressant and add psychotherapy as an augmenting treatment. However, psychotherapy may not always be available, and many patients decline it. A meta-analysis of 6 randomized trials compared antidepressants plus add-on psychotherapy with antidepressants alone in 635 patients with TRD. Remission rate was nearly twice as likely with adjunctive psychotherapy (relative risk, 1.9; 95% CI, 1.5-2.5), and discontinuation of treatment was comparable for the 2 groups.20 As telepsychiatry and telemedicine become more common in clinical practice, access to psychotherapy may improve.
Neuromodulation and Interventionals
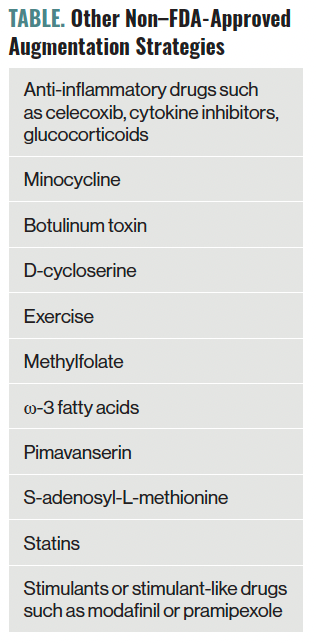
Neuromodulation refers to chemical or electrical interventions that directly alter the nervous system’s function. Neuromodulatory treatments tend to be focal in nature, directly modifying the activity of discrete areas in the brain or systems and leading to increased neuroplasticity.21 Additionally, neuromodulatory treatments are characterized by their speed of action and cost-effectiveness for the treatment of depression.22 This section will discuss the most common and novel neuromodulatory techniques as augmentation treatments for TRD. Other non–FDA-approved augmentation strategies can be found in the Table.
Table. Other Non–FDA-Approved Augmentation Strategies
Electroconvulsive Therapy
Electroconvulsive therapy (ECT) has been used for the past 80 years and has robust evidence for TRD treatment, displaying a 64% response rate and 48% remission rate in individuals with TRD. ECT is superior to pharmacotherapy for treating depression23 and is associated with reduced psychiatric hospital readmissions.24 Despite its high efficacy rate, ECT’s use has been limited due to its previous negative depictions in popular culture and the associated negative connotation.
ECT is generally safe, yet it is associated with adverse events such as headaches, muscle aches, and cognitive adverse effects. Anterograde amnesia and memory loss can be associated with ECT; however, they resolve within a few weeks of finishing an ECT course, and cognitive function is further improved from baseline thereafter.25 Additionally, these cognitive adverse effects last longer in older adults or when using bitemporal electrode placement. Further advancements in ECT techniques have led to improvements over the years and decreased ECT-related adverse events, including cognitive outcomes and improving patient experience.
Repetitive Transcranial Magnetic Stimulation
Large controlled trials led to FDA approval of repetitive transcranial magnetic stimulation (rTMS) in the treatment of TRD in 2011.26
In patients with TRD, rTMS stimulation of the left dorsolateral prefrontal cortex has resulted in significant improvement of depressive symptoms.26 In addition, rTMS is used as an adjunct treatment to pharmacotherapy and was shown to increase the likelihood of response and remission of symptoms. The durability of rTMS has been exhibited at 12 months’ follow-up after acute treatment.27 A meta-analysis of 4 randomized trials (213 patients) compared high-frequency repetitive TMS plus antidepressants (primarily selective serotonin reuptake inhibitors) with antidepressants alone as initial treatment and found that remission occurred more often with combination treatment (odds ratio, 2.4; 95% CI, 1.3-4.6).28
TMS has several advantages: It does not require general anesthesia and has no cognitive adverse effects due to its focal administration. This is particularly important for older adults with TRD, as it has a benign cognitive profile compared with ECT.
Ketamine
Ketamine is an anesthetic agent with N-methyl- D-aspartate receptor (NMDAR) blockade properties that has more recently been shown to be a promising and novel augmentation agent for the treatment of depression. Multiple studies have confirmed the efficacy of intravenous ketamine for TRD.29 Intravenous (IV) ketamine is given at subanesthetic doses of 0.5 mg/kg over a 40-minute infusion, typically 2 or 3 times a week over 3 to 4 weeks. Esketamine, the intranasal form, received FDA approval in 2019 for treatment of depression. Although IV ketamine appears to be more efficacious than esketamine,30 it is rarely covered by insurance and costs are covered by patients out of pocket. To further support ketamine, a recent open-label, randomized, noninferiority trial (NCT03113968) compared treatment response in 403 participants with TRD who received IV ketamine or ECT.31 The results of this study found IV ketamine to be noninferior to ECT based on the primary outcome being response to treatment, which was defined as a greater than 50% reduction in score on the Quick Inventory of Depressive Symptomology scale. Furthermore, the participants in the IV ketamine arm had fewer cognitive adverse effects and decline in memory recall compared with the ECT group.
Dr Madan is an assistant professor in the Department of Psychiatry at the University of Arizona College of Medicine in Tucson. Dr Oughli is an assistant professor in the Department of Psychiatry and Biobehavioral Sciences at the University of California, Los Angeles. Dr Gebara is an assistant professor in the Department of Psychiatry at the University of Pittsburgh School of Medicine in Pennsylvania.
References
1. Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol. 2007;17(11):696-707.
2. Fekadu A, Donocik JG, Cleare AJ. Standardisation framework for the Maudsley staging method for treatment resistance in depression. BMC Psychiatry. 2018;18(1):100.
3. Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry. 1997;58(suppl 13):23-29.
4. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
5. Trevino K, McClintock SM, McDonald Fischer N, et al. Defining treatment-resistant depression: a comprehensive review of the literature. Ann Clin Psychiatry. 2014;26(3):222-232.
6. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
7. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):179-200.
8. Zhdanava M, Pilon D, Ghelerter I, et al. The prevalence and national burden of treatment-resistant depression and major depressive disorder in the United States. J Clin Psychiatry. 2021;82(2):20m13699.
9. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917.
10. Buchalter ELF, Oughli HA, Lenze EJ, et al. Predicting remission in late-life major depression: a clinical algorithm based upon past treatment history. J Clin Psychiatry. 2019;80(6):18m12483.
11. Rush AJ, Trivedi MH, Wisniewski SR, et al; STAR*D Study Team. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354(12):1231-1242.
12. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318(2):132-145.
13. Lenze EJ, Mulsant BH, Roose SP, et al. Antidepressant augmentation versus switch in treatment-resistant geriatric depression. N Engl J Med. 2023;388(12):1067-1079.
14. Tran K, McGill SC, Horton J. Bupropion for treatment-resistant depression. Canadian Agency for Drugs and Technologies in Health; 2021.
15. de Boer T. The pharmacologic profile of mirtazapine. J Clin Psychiatry. 1996;57(suppl 4):19-25.
16. Bauer M, Adli M, Bschor T, et al. Lithium’s emerging role in the treatment of refractory major depressive episodes: augmentation of antidepressants. Neuropsychobiology. 2010;62(1):36-42.
17. Ostroff RB, Nelson JC. Risperidone augmentation of selective serotonin reuptake inhibitors in major depression. J Clin Psychiatry. 1999;60(4):256-259.
18. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
19. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980-991.
20. Ijaz S, Davies P, Williams CJ, et al. Psychological therapies for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2018;5(5):CD010558.
21. Abbott CC, Jones T, Lemke NT, et al. Hippocampal structural and functional changes associated with electroconvulsive therapy response. Transl Psychiatry. 2014;4(11):e483.
22. Ross EL, Zivin K, Maixner DF. Cost-effectiveness of electroconvulsive therapy vs pharmacotherapy/psychotherapy for treatment-resistant depression in the United States. JAMA Psychiatry. 2018;75(7):713-722.
23. UK ECT Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799-808.
24. Slade EP, Jahn DR, Regenold WT, Case BG. Association of electroconvulsive therapy with psychiatric readmissions in US hospitals. JAMA Psychiatry. 2017;74(8):798-804.
25. Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry. 2010;68(6):568-577.
26. Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549-1560.
27. Dunner DL, Aaronson ST, Sackeim HA, et al. A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry. 2014;75(12):1394-1401.
28. Berlim MT, Van den Eynde F, Daskalakis ZJ. High-frequency repetitive transcranial magnetic stimulation accelerates and enhances the clinical response to antidepressants in major depression: a meta-analysis of randomized, double-blind, and sham-controlled trials. J Clin Psychiatry. 2013;74(2):e122-129.
29. Marcantoni WS, Akoumba BS, Wassef M, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 - January 2019. J Affect Disord. 2020;277:831-841.
30. Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542-555.
31. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023;388(25):2315-2325.

