|Slideshows|May 13, 2019
7 Ways to Treat Tardive Dyskinesia
Author(s)Chris Aiken, MD
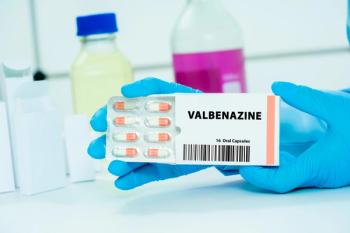
Once thought untreatable, we now have two FDA-approved medications for TD and a handful of off-label options. More in this research update.
Advertisement
Newsletter
Receive trusted psychiatric news, expert analysis, and clinical insights — subscribe today to support your practice and your patients.
Advertisement
Latest CME
Advertisement
Advertisement
Trending on Psychiatric Times
1
Depression and Long COVID Outcomes in Women: New Data Analysis
2
Presenting Our February 2026 Theme: Bipolar Disorder
3
Investigational Psilocybin for PTSD and Ongoing Trials
4
Patterns of Propensity: A Review of Heinrichs’ How Psychiatrists Make Decisions
5