Major Depressive Disorder
Latest News
Latest Videos

Podcasts
CME Content
More News

A phase 2 study reveals osavampator significantly reduces depression severity in adults with major depressive disorder, showing promise for treatment-resistant cases.

Apathy significantly affects cognitive impairment patients, yet remains underrecognized. Discover effective assessment and treatment strategies for improved outcomes.

New phase 2a study reveals promising results for BPL-003, with a 2-dose regimen improving outcomes for patients with treatment-resistant depression.

New phase 3 data reveals seltorexant's potential as a safer adjunctive treatment for major depressive disorder with insomnia, despite not meeting its primary end point.

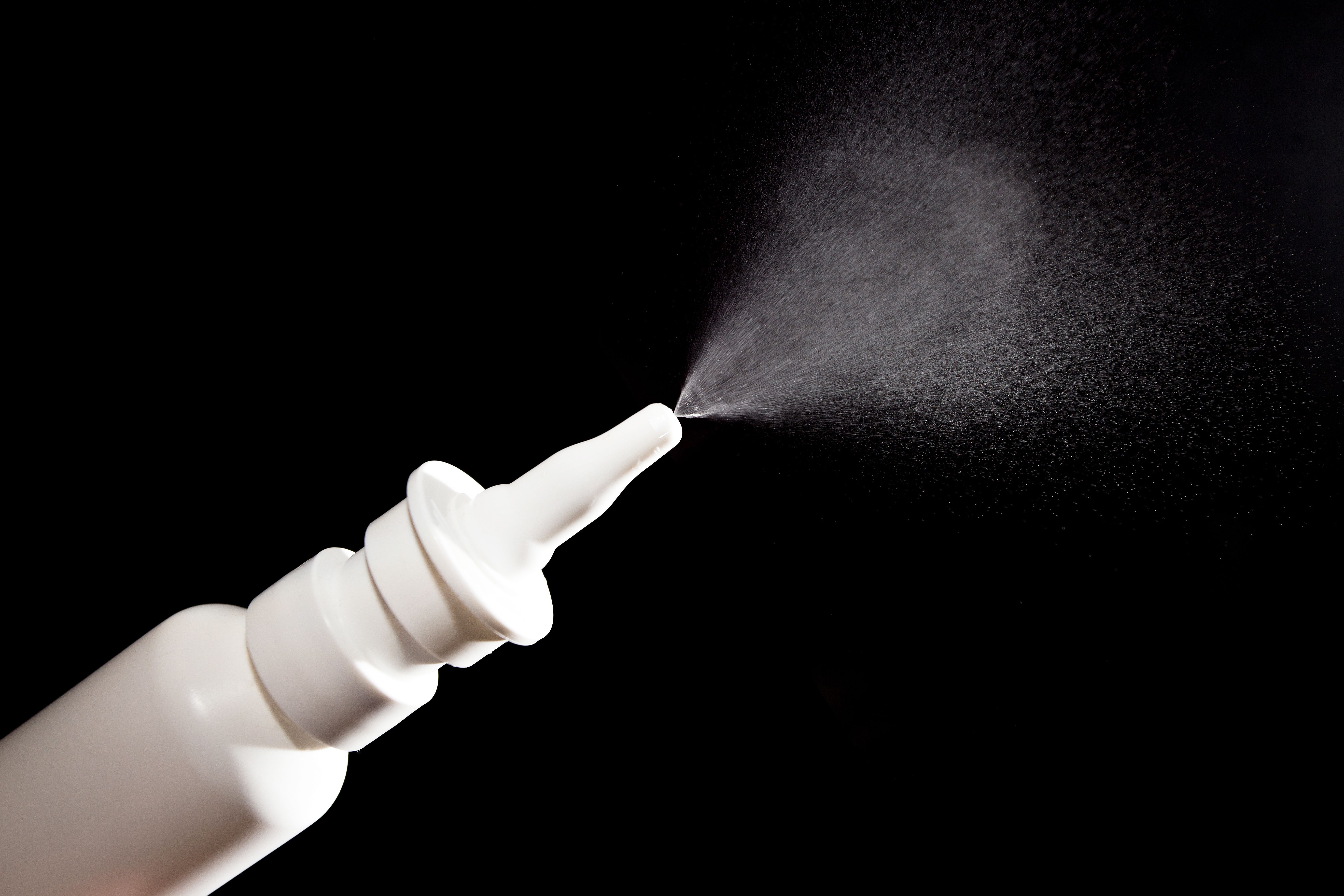
Esketamine nasal spray shows significant improvement in emotional blunting for treatment-resistant depression, offering hope for affected patients.

New study shows lumateperone (Caplyta) significantly improves symptoms in major depressive disorder with anxious distress, offering hope for treatment-resistant patients.

A groundbreaking trial reveals ANK3 as a potential genetic predictor for liafensine's effectiveness in treating resistant depression.

Onfasprodil shows promise as a rapid treatment for resistant depression, offering fewer side effects than ketamine in recent clinical trials.

FDA clears accelerated deep TMS protocol for major depressive disorder, offering faster treatment with similar outcomes and fewer visits.

What is the role of GLP-1 agonists in psychiatry? Joseph F. Goldberg, MD, and Roger S. McIntyre, MD, FRCPC, share their thoughts.

It is time to redefine treatment resistance, a definition in which we emphasize comprehensive evaluations and personalized approaches for better patient outcomes.

Untreated maternal mental health issues during pregnancy pose significant risks. Informed discussions on medication are crucial for mother and baby's well-being.

Antidepressant treatment for late-life depression enhances cognitive functions like memory and learning, linking symptom relief to cognitive improvement.

In his brand new video series, Joseph F. Goldberg, MD, sits down with Marlene Freeman, MD, to discuss the issue of SSRIs and pregnancy.

NRx Pharmaceuticals secures FDA Fast Track designation for NRX-100, a preservation-free formulation of intravenous ketamine for suicidal ideation in depression.

Stay updated on key psychopharmacology developments, including new treatments for schizophrenia and breakthroughs in depression therapy.

Youth with neurodevelopmental disorders face significant suicide risks.

Explore the latest findings on neurotransmitter levels and innovative treatments for major depressive disorder, including TMS and cognitive impacts.

New analysis qualifies OPTIMUM study, finding antidepressant combinations more effective than switching for treatment resistant depression in elderly.

In this CME article, discover effective strategies for prescribing MAOIs in treating major depressive disorder.

Ketamine-assisted psychotherapy has transformative potential as a groundbreaking approach to mental health that enhances healing through psychedelic experiences.

Seaport Therapeutics initiates a pivotal study for GlyphAllo, a potential breakthrough treatment for major depressive disorder, with or without anxious stress.
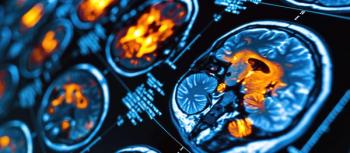
A groundbreaking study reveals that reduced cortical thickness in the parahippocampus may indicate major depressive disorder and neuroticism traits.

Yvette Elpidio, PMHNP-BC, shares insights on NMDA agonists at the Southern California Psychiatry conference.

Fava beans emerge as a powerful natural source of L-DOPA, offering potential benefits for mood disorders and Parkinson's disease and holding enriching culinary traditions.