News
Article
BIAFAC and Posttraumatic Gut-Brain Axis Dysfunction
Author(s):
Brain injury associated fatigue and altered cognition has been associated with a subset of individuals with mild traumatic brain injuries who develop posttraumatic hypopituitarism, including altered gut microbiome and amino acid utilization.
visio/AdobeStock
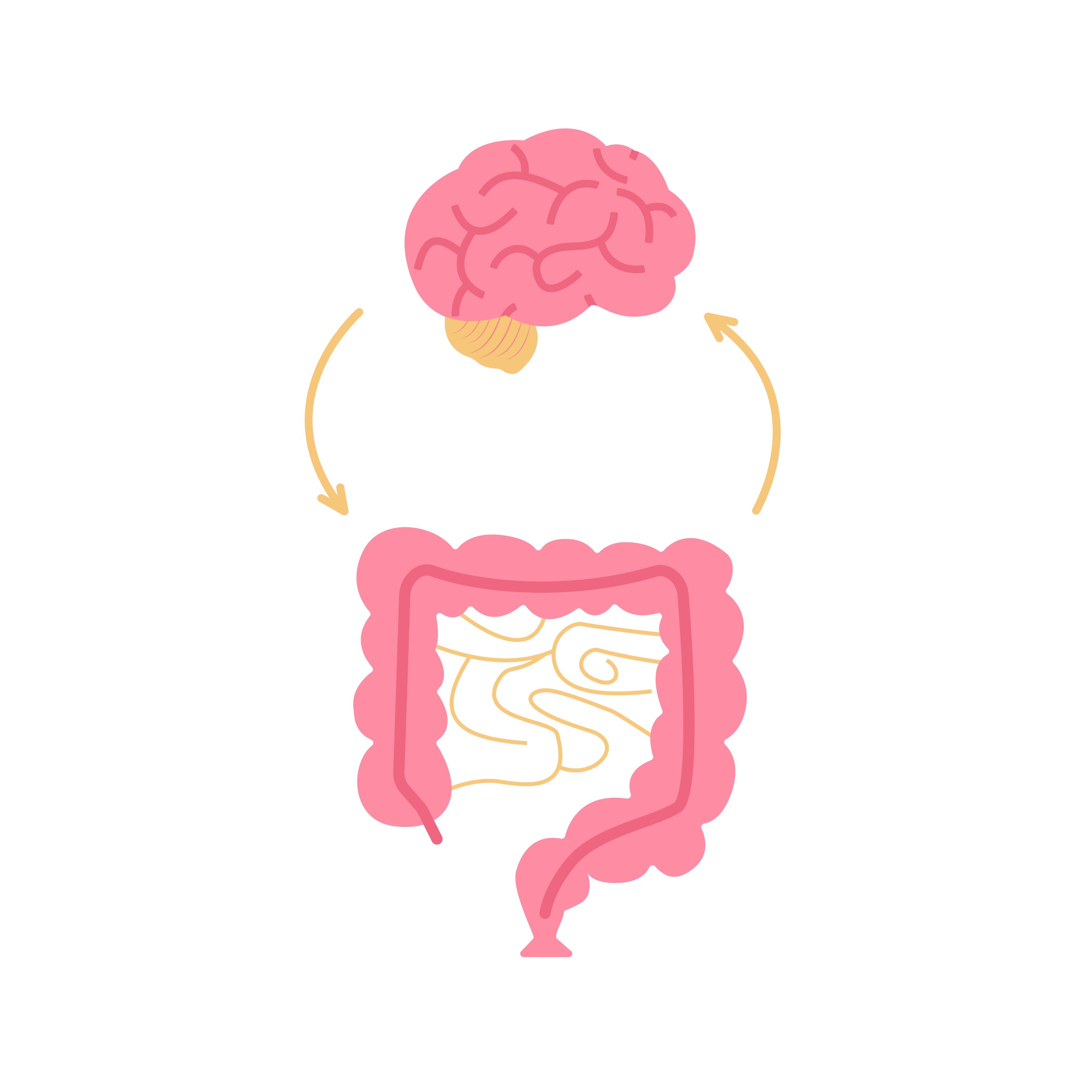
Brain injury associated fatigue and altered cognition (BIAFAC) is characterized by profound fatigue (not ameliorated by sleep), altered cognition (predominantly short-term memory loss and altered executive functioning capabilities), abnormal growth hormone (GH) stimulation test, and a positive response to GH replacement. This condition has been associated with a subset of individuals with mild traumatic brain injuries (TBI) who develop posttraumatic hypopituitarism (PTHP), a condition characterized by a decrease in function of the pituitary gland, and other metabolic abnormalities, including altered gut microbiome and amino acid utilization.1
Similar complaints are common to post-concussion syndrome; however, the symptoms of BIAFAC tend to be delayed, generally not appearing until at least 6 months post-injury. Improvements in fatigue and cognition have been seen following GH replacement, with fatigue improving at about 3 months and cognition improving in about 5 to 6 months. When GH replacement is stopped, symptoms return (fatigue returns in approximately 3 months and cognitive impairment at around 6 months).2 This article aims to provide an introduction to the role of amino acids and disruptions in the gut-brain axis in BIAFAC symptoms post-brain injury.
Role of Amino Acids and Neuroendocrine Dysfunction
Amino acids are the building blocks of our bodies and can be broken down into 2 categories, essential and nonessential amino acids. Essential amino acids are those which must be ingested through food, while nonessential amino acids are synthesized by our bodies. Though they are referred to as nonessential amino acids, this is a misnomer, as they are indeed essential to our bodily functions. In the body, amino acids are the building blocks of proteins. The types and patterns of the amino acids determine the function of each protein they synthesize. Thus, by being responsible for the production of proteins, amino acids help in breaking down food, growing and repairing bodily tissue, building muscle, boosting the immune system, providing energy, etc.
Another major role they play is producing hormones and neurotransmitters; therefore, hypoaminoacidemia (abnormally low levels of amino acids in the blood), such has been discovered in TBI, can produce profound physical, cognitive, and neurological dysfunction.3 As an example, glutamate, one of the most abundant amino acids, also functions as the primary excitatory neurotransmitter in the central nervous system. In addition, arginine, an essential amino acid, is a precursor for nitric oxide, while tryptophan, another essential amino acid, is a precursor to serotonin. Serotonin and nitric oxide are both tied to cognition and mood.4 Disruption of this delicate balance of amino acids, proteins and neurotransmitters can contribute to the symptoms of BIAFAC seen post-TBI.
Hypoaminoacidemia and Gut Microbiome Dysbiosis in TBI
Studies have shown that an altered gut microbiome may be responsible for amino acid abnormalities after TBI.5
The gut microbiome is unique to each individual and is originally introduced at birth via microbes present at the time of delivery and through formula or breast milk. Later, other microbes are introduced to the biome via our diet and through environmental exposure. The microbiome consists of both helpful and potentially harmful microbes. Bacteria in our gut decide what our body absorbs, what gets flushed out in the feces, and what gets absorbed by bacteria for their own growth. A consistent balance of gut microbiota is required for optimal health.
Literature supports the concept that the gut-brain axis is a functional organ system that includes amino acid signals and short chain fatty acids as part of a bidirectional relationship between the central nervous system and the gut microbiome. It appears that the vagus nerve and the autonomic nervous system in the spinal cord are the conduits for communication between the gut and the brain. Thus, as one might expect, gut dysbiosis has also been found in spinal cord injuries.6
A brain injury triggers countless cellular and molecular processes that may lead to rapid changes in the gut microbiome due to the bidirectional relationship that exists between the brain and the gut. Changes in the gut microbiome include alterations in the motility and permeability of the intestinal wall and activation of immune cells. Studies in both animals and humans have shown that TBI significantly impacts circulating amino acids the amount and diversity of gut bacteria.3,5
Addressing Gut Microbiome Dysbiosis
Studies investigating the post-injury disruption of hormones, amino acids, and the gut microbiome are helping to illustrate TBI as a chronic disease state. Understanding the underlying mechanisms may lead to better treatments and/or prevention of secondary consequences of TBI, including BIAFAC. Some promising interventions include the use of prebiotics, which foster the growth and activity of beneficial microorganisms, ie bacteria and fungi (acting as “food” or energy for the bacteria), probiotics, which are live microorganisms, ie, the bacteria itself, or even the potential of fecal transplants.
Dr Howell is a senior neuroscientist at the Centre for Neuro Skills. She is a specialist in brain injury rehabilitation, neurodegenerative disease, and clinical research.
References
1. Yuen KCJ, Masel BE, Reifschneider KL, et al. Alterations of the GH/IGF-I axis and gut microbiome after traumatic brain injury: a new clinical syndrome? J Clin Endocrinol Metab. 2020;105(9):3054-3064.
2. Urban RJ. A treatable syndrome in patients with traumatic brain injury. J Neurotrauma. 2020;37(8):1124-1125.
3. Durham WJ, Foreman JP, Randolph KM, et al. Hypoaminoacidemia characterizes chronic traumatic brain injury. J Neurotrauma.2017;34(2):385-390.
4. Armstrong PA, Venugopal N, Wright TJ, et al. Traumatic brain injury, abnormal growth hormone secretion, and gut dysbiosis. Best Pract Res Clin Endocrinol Metab. 2023;37(6):1018-1041.
5. Urban RJ, Pyles R, Stewart C, et al. Altered fecal microbiome years after traumatic brain injury. J Neurotrauma. 2020;37(8):1037-1051.
6. Jing Y, Bai F, Yu Y. Spinal cord injury and gut microbiota: a review. Life Sci. 2021;266:118865.
Newsletter
Receive trusted psychiatric news, expert analysis, and clinical insights — subscribe today to support your practice and your patients.






