Tardive Dyskinesia
Latest News
Latest Videos

CME Content
More News

What do we know about TD? What is on the horizon? Take the quiz to learn more.

How could drugs that block post-synaptic dopamine 2 receptors increase the risk for tardive dyskinesia over time?

Major risk factors for onset of tardive dyskinesia are considered.

What major challenges do patients with tardive dyskinesia face in their daily lives? Take the quiz and learn more.

The first study to gather unsolicited patient responses via social media finds 3 negative themes.
Learn about the results of the recent study on deutetrabenazine and the ways it is helping patients with tardive dyskinesia.

There are a few things that we all agree on, as reflected in a recent consensus statement.

Numbers don’t tell the whole story, but gaining a better sense of the frequency, risks, and impact on patients’ quality of life can help improve treatment efforts and enhance outcomes.

There are ways to prevent, manage, and treat TD, and this podcast will guide you through those steps from FDA-approved medications to off-label therapies.

Once thought untreatable, we now have two FDA-approved medications for TD and a handful of off-label options. More in this research update.

Tardive dyskinesia hits affected populations hard, especially older folks with poor support systems.

Recent studies and reviews report great progress in bringing TD under control.

Some dyskinesias improve with antipsychotic treatment, and these types are more common than thought. More in this podcast.

A prediction model may help identify treatment characteristics associated with TD among patients with psychiatric disorders taking antipsychotic medications.

This video will help clinicians detect tardive dyskinesia and movement disorders in patients taking antipsychotic drugs.

In this quiz, learn which medical disorders patients with schizophrenia and comorbid TD are likely to contend with.

A program guide for the Abnormal Involuntary Movement Scale video.

Tardive syndromes include a broad spectrum of abnormal movements. Which movement disorders can resemble tardive dyskinesia? Find out in the quiz.
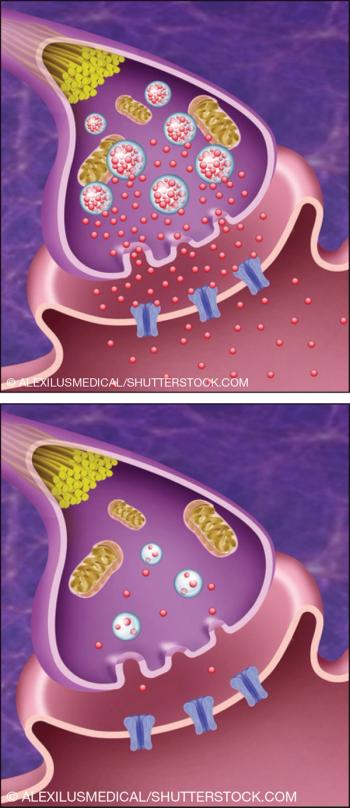
We’ve been waiting since 1953, the year chlorpromazine was introduced to the US as a revolutionary treatment for schizophrenia, for an active treatment for tardive dyskinesia that the FDA judged to be effective.

The results of a phase 3 trial demonstrated significant benefit over placebo.
A reader asks an expert about differential features distinguishing between early stages of tardive dyskinesia and motor tics.